Tofacitinib API: Mechanism, Clinical Uses, and Formulation Insights
Tofacitinib API: Mechanism, Clinical Uses, and Formulation Insights
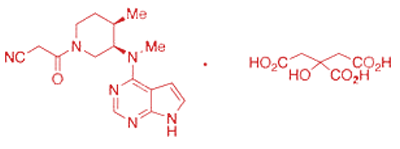
Tofacitinib Citrate API is a Janus kinase (JAK) inhibitor used to manage autoimmune diseases such as rheumatoid arthritis, psoriasis, and ulcerative colitis. As a small-molecule immunomodulator, it interrupts cytokine signalling by inhibiting the JAK-STAT pathway.
With the rising demand for oral biologic alternatives, Tofacitinib Citrate API has become one of the most sought-after molecules among global B2B buyers. This guide explores its pharmacology, formulation, regulatory considerations, and why Bio-Synth a Hyderabad-based WHO-GMP-certified organisation, stands among the top Tofacitinib Citrate API Manufacturers in India.
Understanding Tofacitinib: A Potent Kinase Inhibitor API
What is Tofacitinib?
Tofacitinib Citrate API is a synthetic small molecule classified under Janus kinase (JAK) inhibitors. It modulates immune responses by targeting intracellular signaling enzymes that regulate cytokine activity.
Quick Facts:
- API Category: Kinase inhibitor (JAK family)
- Molecular Formula: C₁₆H₂₀N₆O
- Therapeutic Class: Immunomodulator
- Regulatory Status: Approved by FDA, EMA, and DCGI
- Supplied As: High-purity crystalline API powder
How It Works: JAK-STAT Pathway Inhibition
Tofacitinib Citrate API inhibits JAK enzymes, disrupting cytokine signalling via the JAK-STAT pathway — a key driver of inflammation.
| Component | Function | Role of Tofacitinib |
| Cytokines | Trigger immune response | Reduces cytokine sensitivity |
| JAK enzymes | Transmit immune signals | Direct enzyme inhibition |
| STAT proteins | Activate inflammatory genes | Blocks gene activation |
| Immune response | Maintains inflammation balance | Downregulated |
By preventing cytokine activation, Tofacitinib Citrate API effectively reduces chronic inflammation in autoimmune conditions.
Clinical Applications of Tofacitinib API
Approved Therapeutic Indications:
- Rheumatoid Arthritis (RA) – as monotherapy or with methotrexate
- Psoriatic Arthritis – for patients unresponsive to biologics
- Ulcerative Colitis – oral alternative to injectable biologics
Investigational/Off-label Uses: Alopecia areata, lupus, and chronic plaque psoriasis
Formulation Routes:
- Oral Tablets: 5 mg and 10 mg
- Extended-Release Tablets: 11 mg once daily
- Combination Formulations: Under research with methotrexate and biologics
Its pharmacokinetics support once- or twice-daily dosing for improved patient adherence.
Why Tofacitinib Matters for Pharma Buyers and CDMOs
Rising Global Demand
The autoimmune therapeutics market is projected to exceed $153 billion by 2030, driven by demand for oral biologics such as Tofacitinib Citrate API.
Key Benefits:
- Convenient oral administration
- Long shelf life suited for export markets
- Fast-acting with visible clinical improvement in weeks
Importance of High-Purity API
Pharma formulators require:
- ≥ 99% purity (USP / Ph.Eur compliant)
- Minimal residual solvents
- ICH-zone stability data
- WHO-GMP and regulatory documentation
The synthesis complexity of Tofacitinib Citrate API demands precision in impurity profiling and crystallization control.
🔍 Explore Bio-Synth’s Tofacitinib Citrate API Manufacturers and Suppliers in India for GMP-compliant kinase inhibitors and high-purity actives.
Manufacturing Insights: Indian Advantage in API Production
India’s API Leadership
India ranks among the top three API exporters globally, offering:
- Cost-efficient CDMO capabilities
- WHO-GMP-certified facilities
- Regulatory expertise with USFDA, EMA, PMDA
Why Hyderabad is the API Hub
Hyderabad’s Balanagar Industrial Area is home to advanced pharmaceutical manufacturers supported by:
- Skilled process chemists and engineers
- Regulatory filing expertise (DMF, CEP, ANDA)
- Strong logistics and export infrastructure
Bio-Synth’s Manufacturing Excellence
Founded in 1943, Bio-Synth has decades of experience in API synthesis and contract manufacturing.
Key Capabilities:
- Over 50+ APIs and intermediates developed
- Partnerships with leading global pharmaceutical companies
- Advanced purification and crystallization technologies
- APIs compliant with ICH Q7 and WHO-GMP standards
Regulatory and Supply Chain Excellence
Comprehensive Documentation
Each Tofacitinib Citrate API batch includes:
- Drug Master File (DMF) – US & EU
- Certificate of Analysis (CoA)
- Batch Manufacturing Record (BMR)
- Stability Data (ICH Zone I–IV)
These streamline regulatory submissions for ANDA or CTD filings.
Global Export Capabilities
Bio-Synth operates from Hyderabad SEZs with:
- Ambient and cold-chain logistics
- Flexible pack sizes (10 g – 50 kg)
- Fast air and sea dispatches to North America, MENA, CIS, and Southeast Asia
Process Flow: From Lab to Dispatch
Synthesis → Purification → QC Testing → Regulatory Filing → Packaging → Export Dispatch
| Stage | Focus Area |
| Synthesis | Multi-step organic process |
| QC Testing | HPLC, NMR, residual solvent analysis |
| Regulatory | DMF, CoA, stability reports |
| Packaging | Triple LDPE liners in HDPE drums |
| Export | WHO-GMP-certified batch dispatch |
B2B Buyer’s Sourcing Checklist
When sourcing Tofacitinib Citrate API Suppliers in India, verify:
- WHO-GMP / USFDA audit status
- DMF availability
- Three-batch analytical data
- ICH impurity profiling
- Market-specific regulatory support
A supplier like Bio-Synth provides full technical transparency and documentation, making it one of the most reliable Tofacitinib Citrate API Manufacturers and Suppliers globally.
Smart Formulation Insights
R&D and formulation teams should evaluate:
- Solubility: Moderate; requires excipient optimisation
- pKa: ~5, affecting absorption kinetics
- Stability: Requires photostability testing
- Polymorph Control: Ensures bioequivalence consistency
These parameters make a reliable Tofacitinib Citrate API Manufacturer essential for formulation success.
FAQs: Buyer & Scientist Quick Answers
Q1: Why do global buyers prefer Indian API suppliers for kinase inhibitors?
India offers cost-efficient synthesis, WHO-GMP compliance, and complete regulatory documentation. Hyderabad’s industrial ecosystem supports rapid, export-grade production.
Q2: How does Tofacitinib differ from biologics?
Unlike injectable biologics, Tofacitinib Citrate API is a small molecule oral drug that targets intracellular JAK enzymes, offering similar efficacy with higher patient convenience.
Q3: Can Indian CDMOs manufacture Tofacitinib for regulated markets?
Yes. CDMOs like Bio-Synth follow cGMP protocols, provide DMF support, and ensure flexible batch sizes for both regulated and semi-regulated markets.
Conclusion:
Partner with a Trusted Indian API Manufacturer
Tofacitinib Citrate API is revolutionising immunology by offering an oral, cost-efficient alternative to injectable biologics. For B2B buyers and formulation partners, sourcing from WHO-GMP-certified Tofacitinib Citrate API Manufacturers in India ensures reliability and regulatory readiness.
With over 80 years of heritage, Bio-Synth continues to deliver high-purity Tofacitinib Citrate API with global quality standards and efficient export logistics.
📩 Contact Bio-Synth for quotes, DMF access, or CDMO collaboration opportunities.